Dr Rick Suenram at NIST in the 1980s and 90s was looking deeply, expensively into this, involving exposing inert gases inside high-vacuum chamber to high intensity microwave RF (minus the requisite particle radiation, this was essentially HUTCHISON EFFECT research but on gases not solids). Research echoed Exactly what was described (revealed?) decades later by David Wilcox (new-ager) as “LOOKING GLASS” technology — the see-into-the-future tech / gizmo. The research at NIST was very expensive, lasted decades, and was taken Very seriously. Many barely-sciencey / non-occultist types knee-jerk ‘laughed off’ the research, until highly interesting reproducible data began to surface…
Platinum Hexafluoride, the most-powerful oxidizing agent ever discovered (1962).
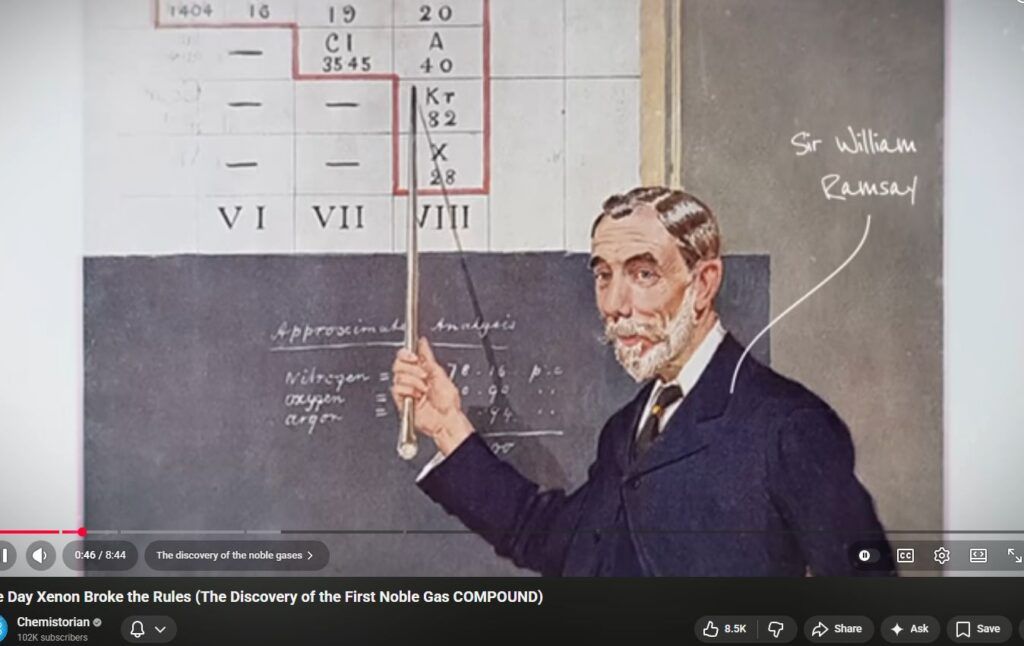
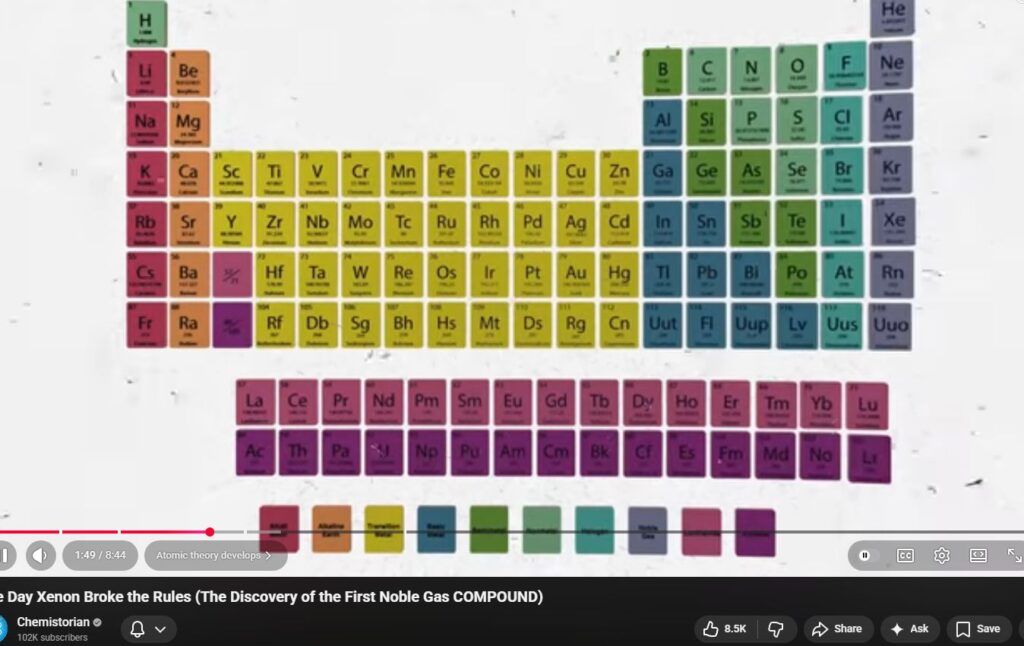
Richard D. Suenram’s published work and associated NBS/NIST program documentation describe two different experimental contexts in which noble gases are present under vacuum and microwaves/RF are used. The observed “reactivity” depends on which context is meant: (1) formation of weakly bound rare-gas complexes detected by microwave spectroscopy, or (2) chemistry enabled by microwave/RF discharges that create excited/metastable noble-gas species and electrons.[2][3][4]
1) High-vacuum microwave spectroscopy: noble gases as complex-forming partners and beam carriers
Experimental setup used in Suenram-era NBS/NIST FTMW work
In pulsed-beam Fourier-transform microwave (FTMW) spectroscopy, a Fabry–Perot cavity is housed in a high-vacuum chamber, and a pulsed supersonic expansion introduces a molecular beam into the cavity region.[3] Noble gases (Ne, Ar, Kr) are commonly used as the carrier gas to cool the expansion and stabilize weakly bound species long enough for spectroscopic detection.[3]
What “reactivity” means in this regime
In this high-vacuum FTMW context, “reactivity” is typically not the formation of new covalent compounds by noble gases. Instead, it is the formation of van der Waals (weakly bound) complexes between a noble gas atom and a polarizable molecule, which become observable because the jet expansion cools rotational motion and reduces collisional breakup.[3]
Representative rare-gas complex targets
Suenram coauthored work reporting FTMW measurements and structural analyses for complexes such as OCS with Ne/Ar/Kr (Ne–OCS, Ar–OCS, Kr–OCS), including isotopologues, with spectra assigned and used to infer geometry and intermolecular parameters.[3]
Role of the microwave/RF field in this regime
In the FTMW cavity experiment, the microwave field is used to excite and measure rotational transitions of neutral molecules/complexes.[3] The field functions as a spectroscopic probe rather than as an ionizing or chemistry-driving energy source in the way a discharge does.[3]
2) Microwave/RF discharge chemistry: noble gases as energetic agents (via excited/metastable states)
Microwave discharge as a chemical production method
Separately from cavity FTMW measurements, Suenram and colleagues published and are cited in NIST documentation for using microwave discharges to generate reactive products (radicals, transient species) and then identifying them spectroscopically.[1] In this regime, microwave power sustains a plasma; electrons and excited species drive bond breaking/forming pathways that do not occur in the neutral, cold-jet FTMW regime.[1]
Excited noble-gas atoms used to drive reactions (matrix isolation context)
NBS/NIST program reports describe experiments in which argon atoms excited in a low-power microwave discharge were used as an energetic reactant/activator in codeposition experiments to generate otherwise difficult-to-form intermediates (example reported: trans-HOCO generated via codeposition involving an Ar:HCOOH sample and a beam of discharge-excited argon atoms).[4] This is a specific case where argon is not acting only as an inert diluent; the “reactive” role is tied to argon’s electronically excited population created by the discharge.[4]
Noble-gas discharge enabling ion formation in a noble-gas matrix
NIST historical documentation also describes experiments where neon subjected to a microwave discharge was co-deposited with an acetylene–neon mixture at cryogenic temperature, with spectral evidence interpreted as formation of an acetylene cation in solid neon.[2] This again attributes chemical activation to discharge-produced energetic species rather than to ground-state neon chemistry.[2]
Mechanistic framing commonly used for “noble-gas reactivity” in discharges
In low-pressure plasmas, noble gases can populate long-lived metastable excited states that transfer energy to other molecules by collision, including pathways such as Penning ionization when energetically allowed.[5] This is the standard physical basis for describing noble gases as chemically “activating” in discharge environments.[5]
3) How to map “high vacuum + microwave/RF impingement” to Suenram-linked results
Under high vacuum with microwave fields used for FTMW spectroscopy, the prominent noble-gas-associated phenomenon documented in Suenram’s coauthored work is observation and characterization of rare-gas van der Waals complexes (e.g., Ne/Ar/Kr–OCS) rather than covalent noble-gas chemistry.[3] Under microwave/RF discharge conditions, NBS/NIST documentation describes noble gases participating indirectly as energetic carriers (excited atoms, metastables, plasma electrons) that enable formation of ions or reactive intermediates in co-deposited or mixed systems.[2][4][5]
Endnotes
[1] NIST Journal of Research (JRES) reference list and historical overview mentioning Suenram/Lovas “Reaction Products from a Microwave Discharge …” series (e.g., N₂ + H₂S discharge products). https://nvlpubs.nist.gov/nistpubs/jres/117/jres.117.016.pdf
[2] NIST historical chapter describing microwave-discharge-involved matrix/cryogenic experiments including neon subjected to microwave discharge and ion evidence in solid neon. https://www.nist.gov/system/files/documents/nvl/SP955_12_CHAPTER_SIX.pdf
[3] “Pulsed Beam Fourier Transform Microwave Measurements on OCS and Rare Gas Complexes of OCS with Ne, Ar and Kr” (Fabry–Perot cavity in high vacuum; rare-gas–OCS complex spectra/structure). https://www.researchgate.net/publication/233927202_Pulsed_Beam_Fourier_Transform_Microwave_Measurements_on_OCS_and_Rare_Gas_Complexes_of_OCS_with_Ne_Ar_and_Kr
[4] NBS report section “Excited Argon-Atom Reaction Studies” describing argon atoms excited in a low-power microwave discharge used in codeposition chemistry (example: trans-HOCO). https://www.govinfo.gov/content/pkg/GOVPUB-C13-8fc88cc554fc1ead1f6fd81575317b1d/pdf/GOVPUB-C13-8fc88cc554fc1ead1f6fd81575317b1d.pdf
[5] Reference overview on Penning ionization and metastable-driven ionization mechanisms in discharge environments. https://www.sciencedirect.com/topics/chemistry/penning-ionization
