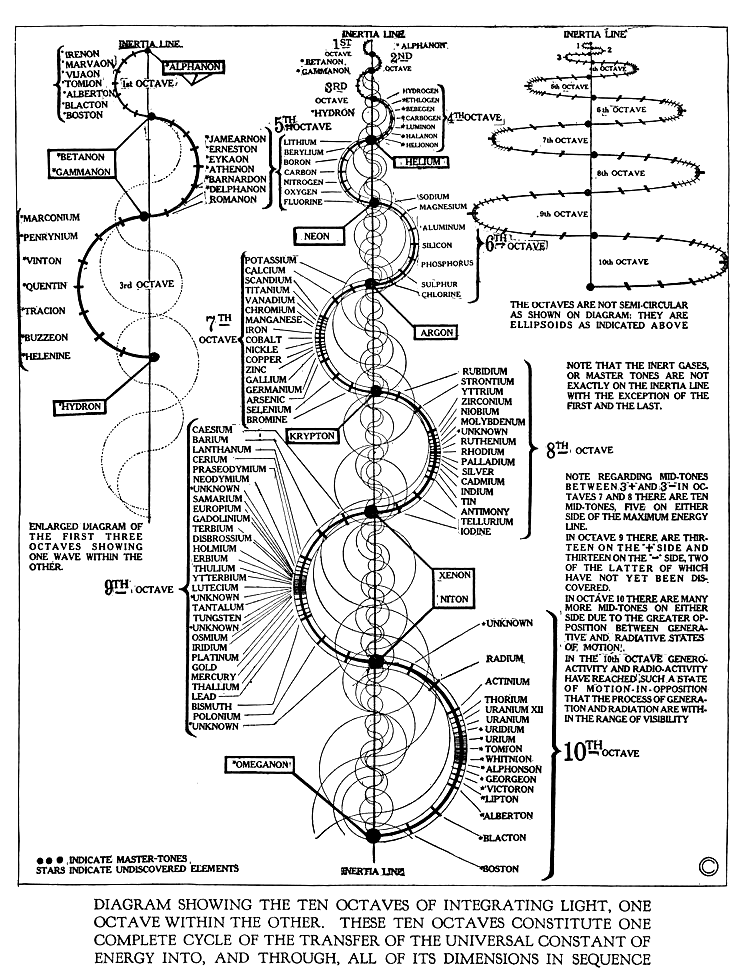
In this theory the elements of the Periodic Table are standing waves over a period of time. Time is continuously being formed by the spontaneous absorption and emission of light waves of EMR.
https://www.youtube.com/watch?v=qhg2uOSb-LA
The Spiral Periodic Table, also known as the helical or circular periodic table, is an alternative representation of the traditional periodic table of the elements. This innovative design was conceived to provide a more intuitive visualization of the relationships and properties of the chemical elements, especially highlighting the periodicity and trends that are fundamental to the table’s structure.
### History and Development
The concept of arranging the elements in a spiral form has been explored by several scientists and educators since the discovery of the periodic law by Dmitri Mendeleev in the late 19th century. One of the earliest and most notable versions was proposed by the French geologist Alexandre-Émile Béguyer de Chancourtois in 1862, even before Mendeleev’s periodic table, with his “vis tellurique” (telluric helix), which was a three-dimensional spiral representation of the elements according to their atomic weights.
Over the years, various versions of the spiral periodic table have been developed, each aiming to address specific aspects of chemical element relationships or to provide a more comprehensive understanding of the periodic trends. These designs vary in their complexity and the manner in which they represent the sequence of elements, the transition between periods, and the categorization of elements into groups or families.
### Features and Advantages
– **Periodicity and Trends**: The spiral layout can make it easier to visualize the periodic nature of the elements’ properties, as the continuity of the spiral emphasizes the repetition of chemical behaviors across periods.
– **Group Relationships**: By arranging the elements in a spiral, certain versions of the table can more clearly demonstrate the relationships between different groups of elements, such as the transition metals, lanthanides, and actinides.
– **Educational Tool**: For educational purposes, the spiral periodic table can offer an alternative perspective on the elements, potentially aiding in understanding the underlying principles of periodicity and the structure of the electron shells.
### Variations
There are several variations of the spiral periodic table, with some focusing on the symmetry of electron configurations, while others aim to represent more accurately the elements’ physical and chemical properties in relation to their positions. The design can be a simple flat spiral, a more complex three-dimensional helix, or even a multi-layered spiral, depending on the specific goals of the representation.
### Criticism and Limitations
While the spiral periodic table provides an interesting alternative to the traditional tabular layout, it also faces criticism and limitations. One challenge is that not all versions of the spiral table clearly delineate the groups or families of elements, which can make it difficult to quickly identify elements with similar chemical properties. Additionally, the compact nature of the spiral design might limit the amount of information that can be displayed for each element, compared to the traditional table.
### Conclusion
The Spiral Periodic Table represents a creative approach to visualizing the relationships and properties of the chemical elements. While it may not replace the traditional periodic table in most scientific or educational contexts, it offers a valuable alternative perspective that can enhance understanding of the periodic trends and the overall structure of the table. Its various incarnations reflect ongoing efforts to make the periodic law more accessible and intuitively understandable.