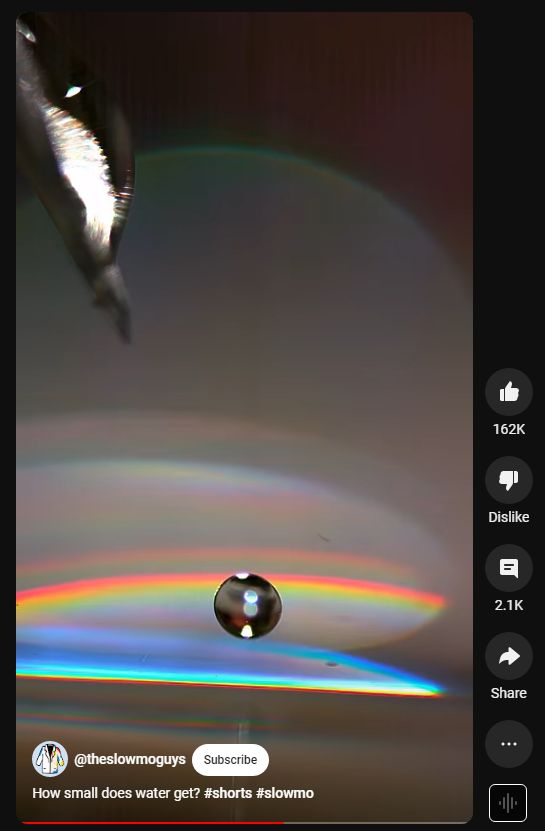
Water droplets at 20k FPS
Surface tension is a phenomenon that occurs at the surface of a liquid, where the molecules are more attracted to each other than they are to the molecules in the air above. This results in the formation of a “skin” or “film” on the surface of the liquid, which gives it the ability to resist external forces.
When a water droplet forms, the water molecules at the surface are attracted to each other, creating surface tension. This surface tension causes the droplet to form a spherical shape, as it minimizes the surface area of the droplet while maximizing the volume it encloses. The spherical shape is the most efficient way for the droplet to contain the greatest volume of water with the least amount of surface area exposed to the air.
Surface tension also affects the behavior of water droplets on surfaces. When a water droplet is placed on a surface, the surface tension causes it to bead up into a spherical shape, rather than spreading out flatly. This is why water droplets on a non-absorbent surface, such as waxed paper or a freshly waxed car, form spherical beads.
Additionally, surface tension enables certain insects, such as water striders, to walk on the surface of water without sinking. The surface tension of the water molecules supports the weight of the insect, allowing it to “float” on the surface tension layer.
Overall, surface tension plays a crucial role in the behavior of water droplets and other liquids, influencing their shape, movement, and interactions with surfaces.